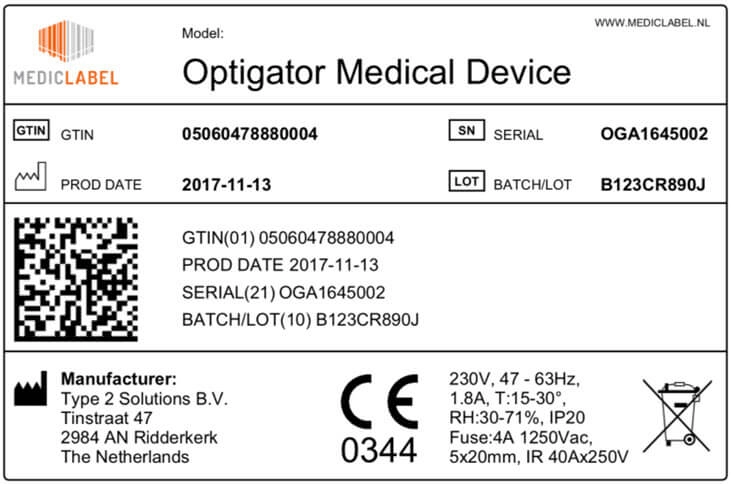
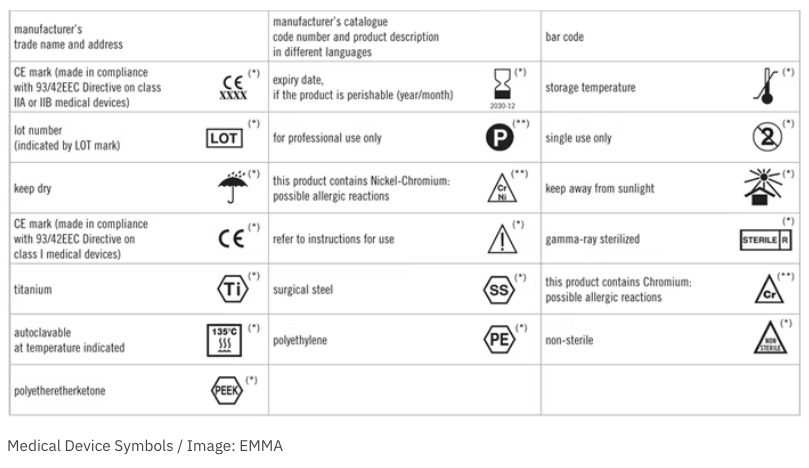
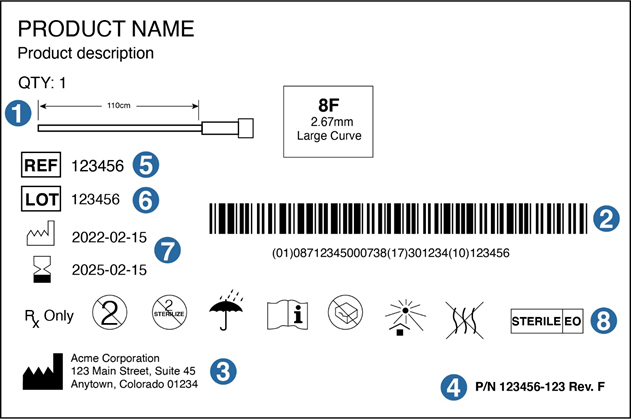
41 fda guidance use of symbols on labels
RoHS Annex 3 Lead Exemptions - RoHS Guide Seven exemption groups have been approved for the use of lead in certain applications under EU RoHS Annex III for a few more years, summarized and detailed below: Lead Category Exemption Deadlines July 21, 2023: Category 8 in-vitro diagnostic medical devices (IVDs) › documents › 2016/06/15Federal Register :: Use of Symbols in Labeling Jun 15, 2016 · All three commenters believed that the Agency's actions in authorizing stand-alone symbols for IVD devices in the guidance document entitled “Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use” (November 2004) (the “IVD Symbols Guidance”) at pp. 7-8 (Ref. 4), and in proposing for this ...
ACADIA Pharmaceuticals Inc. (ACAD) Latest Stock News FDA accepts Acadia's new drug application for Rett syndrome treatment trofinetide SA News Mon, Sep. 12 ACADIA Pharma GAAP EPS of -$0.21 beats by $0.05, revenue of $134.56M beats by $4.5M
Fda guidance use of symbols on labels
Fireworks | PHMSA - Pipeline and Hazardous Materials Safety Administration PHMSA has developed a new IT system which includes a new Fireworks application system. Please visit PHMSA Portal at . This new system will lead to faster processing times while providing more visibility of the application process and easier tracking of the application. During the transition process, there may be some ... How to Identify, Label, Package and Dispose of Biohazardous and Medical ... Biohazardous Waste Disposal Guidelines. Biohazardous waste (e.g., biomedical, infectious, sharps, clinical medical waste, etc.) may be contaminated by blood, body fluids or other potentially infectious materials thus posing a significant risk of transmitting infection to humans or harming the environment. Please follow the guidelines for each ... › medical-devices › device-labelingUse of Symbols in Labeling: Frequently Asked Questions | FDA Manufacturers should look to the final rule, not the withdrawn 2004 guidance, when determining their use of symbols in new labeling and when making labeling updates. 14.
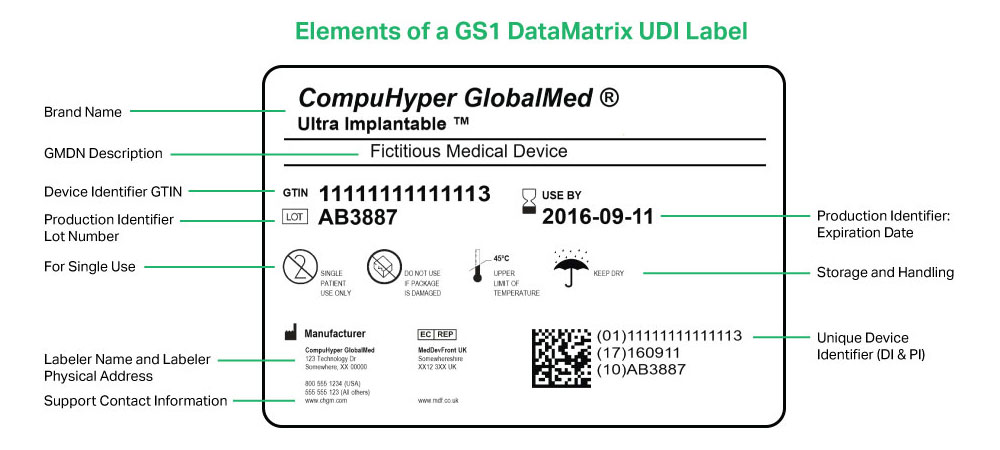
Fda guidance use of symbols on labels. Rivian, Poshmark, Tesla Rise Premarket; Kalvista Pharmceuticals Falls Use standard writing style. Include punctuation and upper and lower cases. Comments that are written in all caps and contain excessive use of symbols will be removed. › documents › 2013/09/24Federal Register :: Unique Device Identification System Sep 24, 2013 · The final rule will permit continued use of an FDA-issued labeler code under an FDA-accredited system for the issuance of UDIs, provided that such use is permitted by the issuing agency that administers that system, and provided the labeler submits a request for continued use of a labeler code; FDA must receive the request no later than ... NASC Quality Seal | NASC LIVE Comply with stringent labeling guidelines for all products and all forms of labeling. Include on product labels any specific warnings and caution statements for particular ingredients that are recommended by the Food & Drug Administration's Center for Veterinary Medicine (FDA-CVM) and the NASC Scientific Advisory Committee. United States Product Labeling Requirements: An Overview - Compliance Gate The Wool Rule stipulates that if a wool product has less than 5% of fiber, the label should state the type and percentage of fiber used, in addition to the percentage of wool or recycled wool used, for example: 98% wool 2% nylon Care Labeling of Textile Wearing Apparel & Certain Piece Goods
FDA Finalizes 'Instructions for Use' Guidance on Patient Labeling for ... [1] Instructions for Use - Patient Labeling for Human Prescription Drug and Biological Products - Content and Format, Guidance for Industry, Food and Drug Admin. (July 2022). (July 2022). eCopy Medical Device Submissions | FDA PDF File Names: Section C of the eCopy guidance describes the 3-digit prefix followed by an underscore that must appear immediately before the Descriptive Name part of the file. You may use any... Gilead Sciences, Inc. (GILD) Latest Stock News | Seeking Alpha Gilead Sciences Non-GAAP EPS of $1.58 beats by $0.06, revenue of $6.26B beats by $390M, raises FY22 EPS guidance. SA NewsTue, Aug. 02 16 Comments. Dietary Guidelines for Americans | health.gov The latest edition of the Dietary Guidelines reflects the current body of nutrition science, helps health professionals and policymakers guide Americans to make healthy food and beverage choices, and serves as the science-based foundation for vital nutrition policies and programs across the United States. News & Announcements
Food Safety / Divisions & Offices / Home - Florida Department of ... Businesses with questions about food safety practices can call the Division of Food Safety at (850) 245-5520 or email FoodSafety@FDACS.gov. Questions about other human health-related impacts of COVID-19 should be referred to the Florida Department of Health's COVID-19 center at 1-866-779-6121 or COVID-19@flhealth.gov. › regulatory-information › search-fdaGuidance for the Use of Bayesian Statistics in Medical Device ... Feb 05, 2010 · You may also send an e-mail request to CDRH-Guidance@fda.hhs.gov to receive a copy of the guidance. Please use the document number 1601 to identify the guidance you are requesting. Meat, Poultry and Egg Product Inspection Directory | Food Safety and ... FSIS is responsible for protecting the public's health by ensuring the safety of meat, poultry, and egg products. FSIS consists of about 9,600 employees, with the majority of agency employees working on the frontline in more than 6,500 federally inspected establishments throughout the United States and Territories, to verify the production of safe, wholesome and properly labeled food. Motion picture content rating system - Wikipedia Yellow - No restrictions: Parental guidance is suggested for designated age range. Purple - No restrictions: Not recommended for a younger audience but not restricted. Red - Restricted: Parental accompaniment required for younger audiences.
IVD companion diagnostics | Therapeutic Goods Administration (TGA) The UPI is the combination of words, numbers, symbols or letters assigned by the manufacturer to uniquely identify an individual IVD (i.e. the device name). Mandatory application audit
› regulatory-information › search-fdaGuidance for Industry, Q7A Good Manufacturing Practice ... Sep 24, 2001 · I. INTRODUCTION (1) A. Objective (1.1) This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs ...
› regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims |FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ...
Valbiotis Accelerates Its Marketing Strategy Our new strategy aims to accelerate the growth of Valbiotis and to boost its capacity for innovation using two main levers: firstly, the signing of international partnerships (global or regional ...
>Understanding a Material Safety Data Sheet (MSDS) Check that the name of the manufacturer and/or supplier matches the label as well. The MSDS and label may also display other identification, such as a product code or catalog number. 2. Hazards Identification The Hazards Identification section describes the ways you may be exposed to the material and the harmful health effects it can have.
FDA Finalizes 'Instructions for Use' Guidance on Patient Labeling for ... The final Guidance suggests that labeling should include a phone number for reporting problems with products and/or reporting adverse events. New language advising applicants to meet with FDA to discuss the IFU during the IND phase. [5] Modification to the title and scope of the Guidance from drug-device combination to drug-led combination ...
Quest Diagnostics (DGX) & Peers Progress With Monkeypox Testing October 3, 2022, 6:35 AM · 3 min read. Quest Diagnostics DGX, just like its industry peers, is progressing in terms of monkeypox virus test development. While the FDA is going all out to increase ...
Kiromic BioPharma Announces FDA Feedback from Type B Pre-IND Meeting ... HOUSTON, October 06, 2022--Kiromic BioPharma, Inc. (NASDAQ: KRBP) ("Kiromic" or the "Company"), a clinical-stage fully-integrated biotherapeutics company using its proprietary DIAMOND® artificial ...
Vietnam | Food Safety and Inspection Service The products identified in the table above are eligible for export from Vietnam to the United States as determined by the United States Department of Agriculture (USDA), Food Safety and Inspection Service (FSIS) Equivalence Process. Vietnam has been allowed to export Siluriformes fish and fish products to the United States under the conditions ...
Television content rating system - Wikipedia Yellow - No restrictions: Parental guidance is suggested for designated age range. Purple - No restrictions: Not recommended for a younger audience but not restricted. Red - Restricted: Parental accompaniment required for younger audiences.
WHMIS 2015 - Safety Data Sheet (SDS) : OSH Answers Section 1 of the SDS should describe the typical use of the product and may indicate restrictions. Ask your supervisor or a health and safety professional for advice if the way you use the product does not match the SDS. Section 2 will summarize the hazards related to the product, precautions to take, and what to do in an emergency.
The FDA Announces A New Definition Of What's 'Healthy' Additionally, the FDA is creating a symbol that businesses can voluntarily use to label food items that adhere to federal definitions of the term "healthy." The statement was made in advance of the White House Conference on Hunger, Nutrition, and Health on Wednesday.
Management of Hazardous Waste Pharmaceuticals | US EPA Guidance Memos and Letters Applicability of 40 CFR Part 266 Subpart P to Intermediate Care Facilities (PDF) (8 pp, 1.29 MB) Chloral hydrate can be U034 (PDF) (11 pp, 56 K) Containers that Once Held P-listed Pharmaceutical Waste (PDF) (6 pp, 1.1 MB) Cyclophosphamide monohydrate can be U058 (PDF) (2 pp, 32 K)
The FDA is updating the definition of 'healthy' and designing new labels The FDA is updating the definition of healthy and designing new labels. The agency says this will help empower people to make better decisions. But not all nutrition experts are convinced.
› food › food-labeling-nutritionUse of the Term Healthy on Food Labeling | FDA The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance.
Custom-made medical devices - Therapeutic Goods Administration (TGA) Sponsors and manufacturers of custom-made medical devices must: notify the TGA they are manufacturing and/or importing custom-made medical devices; adhere to the conditions of exemption relating to inspection and review; adhere to the record keeping requirements; supply their devices with all relevant information required;
FDA Proposes Updated Definition of 'Healthy' Claim on Food Packages to ... Under the proposed definition for the updated "healthy" claim, which is based on current nutrition science, more foods that are part of a healthy dietary pattern and recommended by the Dietary...
FDA's plan to define "healthy" for food packaging: Do we really need it? The FDA has announced the set of rules it proposes to enforce for manufacturers to claim that a food product is "healthy." The proposed rules are a lot better than the labeling anarchy that ...
› medical-devices › device-labelingUse of Symbols in Labeling: Frequently Asked Questions | FDA Manufacturers should look to the final rule, not the withdrawn 2004 guidance, when determining their use of symbols in new labeling and when making labeling updates. 14.
How to Identify, Label, Package and Dispose of Biohazardous and Medical ... Biohazardous Waste Disposal Guidelines. Biohazardous waste (e.g., biomedical, infectious, sharps, clinical medical waste, etc.) may be contaminated by blood, body fluids or other potentially infectious materials thus posing a significant risk of transmitting infection to humans or harming the environment. Please follow the guidelines for each ...
Fireworks | PHMSA - Pipeline and Hazardous Materials Safety Administration PHMSA has developed a new IT system which includes a new Fireworks application system. Please visit PHMSA Portal at . This new system will lead to faster processing times while providing more visibility of the application process and easier tracking of the application. During the transition process, there may be some ...











.png.aspx)

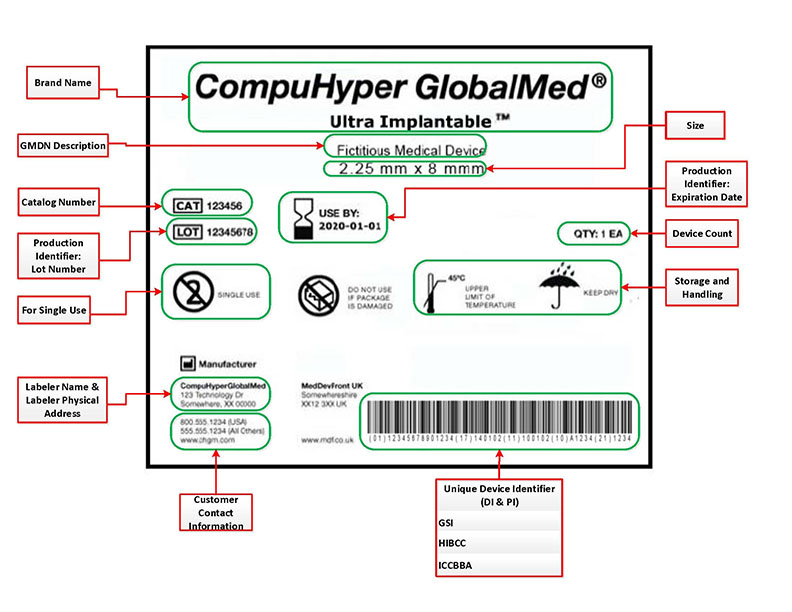










Post a Comment for "41 fda guidance use of symbols on labels"